Citric Acid and Sodium Bicarbonate Reaction Equation
3NaHCO 3aq H 3C 6H 5O 7aq ----- 3CO 2g 3H 2Ol Na 3C 6H 5O 7aq. Web Write a balanced chemical equation for the reaction between sodium carbonate and hydrochloric acid indicating the physical state of the reactants and products.

Solved Problem 1 Citric Acid And Baking Soda Are Used To Chegg Com
Citric acid is an organic weak acid.
. I know that it probably didnt contribute to cancer but could well have as you said fried your fathers kidneys which in turn contributed to perhaps them being that bad enough to go on. As a food preservative Citric acid c. Citric acid can be used in baking powder to react with sodium bicarbonate giving the raising action from carbon dioxide gas formation.
Web When baking soda and citric acid are mixed together with some water they undergo a chemical reaction. Here is the manufacturing process came up by Solvay 1. It has a pH value between 75 and 90.
Web a Acetic acid b Citric acid c Hydrochloric acid d Sulphuric acid. Web Inverted sugar syrup also called invert syrup invert sugar simple syrup sugar syrup sugar water bar syrup syrup USP or sucrose inversion is a syrup mixture of the monosaccharides glucose and fructose that is made by hydrolytic saccharification of the disaccharide sucroseThis mixtures optical rotation is opposite to that of the original sugar which is. Ink stain remover oxalic acid e.
Option c is the answer. Na 2 S 2 O 3. Each unit of blood can generate a total of 23 mEq of bicarbonate as citrate is metabolized.
Web Sodium bicarbonate assists your body in balancing pH levels possibly extending life. Acid react with acids to form salt and water Acid base salt and water HCLNaOH-NaClH2O This reaction is regarded as neutralization reaction as salt formed is neither acidic nor basic its neutral. Web An acid is a molecule or ion capable of either donating a proton ie.
Na 2 CO 3 s 2HCl ag 2NaCl aq CO 2 g H 2 O l Question 2. If 10 g of sodium bicarbonate and 10g citric acid are reacted which is limiting. Web Organic citrate ions are metabolized to bicarbonate ions and result in the buffering of excess hydrogen ions potentially the reversal of acidosis an increase in the plasma bicarbonate concentration and the raising of blood pH.
As eyewash Boric acid b. This reaction is shown in Equation 1 below. Reaction between sodium carbonate Na2CO3 and sodium bisulfite NaHSO3 solution to form the mixed solution of sodium bisulfite and sodium sulfite Na2SO3.
Web In chemistry a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions which results in a compound with no net electric charge. Web The first reaction is the acid-base reaction. The process in which chemical reaction takes place in a solution on passing electricity through it is because of the chemical effect of current.
Do not reveal how the surface area affects reaction rates to your students yet but tell them that they will conduct a series of experiments with Alka-Seltzer tablets in different forms to find. It is produced by citric acid reaction with sodium hydroxide or baking soda. The uses of acids are as follows.
The first category of acids are the proton donors or BrønstedLowry acidsIn the special case of aqueous solutions proton donors form the hydronium ion H 3. Web It is an antioxidant. Web The following is the reaction equation.
Sodium citrate has three forms monosodium citrate disodium citrate and trisodium citrate commonly it refers to last one. Citrates are used in dietary supplements to deliver trace metal minerals in a biologically availableabsorbable chemical form. The result of this initial reaction is two new chemicals.
How much carbon dioxide is produced. A common example is table salt with positively charged sodium ions and negatively charged chloride ions. During summer season a milkman usually adds a small amount of baking.
Web In order to understand how a buffer works consider the example of a buffer solution made by dissolving sodium acetate into acetic acid. Web Calcium carbonate is a chemical compound with the formula Ca CO 3It is a common substance found in rocks as the minerals calcite and aragonite most notably as limestone which is a type of sedimentary rock consisting mainly of calcite and is the main component of eggshells gastropod shells shellfish skeletons and pearlsCalcium carbonate is the. Difference Between Solid Liquid And Gas.
Metal salts from citric acid ie. Effect Of Temperature On The Rate Of Reaction Between Sodium Thiosulphate And Hydrochloric Acid. CH 3 COOH while the sodium acetate dissociates in solution to yield the conjugate base acetate ions of CH 3 COO-.
The equation for the reaction is. Acetic acid is as you can tell from the name an acid. The second reaction is a decomposition reaction.
Web Metabolic alkalosis and hypocalcemia result from sodium citrate and citric acid that is added to blood products in storage to prevent coagulation. Web Citric acid formula. On the other hand a conjugate base is what is left over after an acid has donated a proton during a.
Artificial cherry-mixed fruit flavor anhydrous citric acid colloidal silicon dioxide guar gum magnesium stearate sodium benzoate sodium citrate anhydrous sucrose. Give the uses of acids. Web viii Reaction of dilute hydrochloric acid and sodium sulphide Na 2 S 2HCl 2NaCl H 2 S.
Web Sodium citrate is the sodium salt of citric acid. All bases act as electrolyte in aqueous states ie. Carbonic acid and sodium acetate.
Magnesium sulphate sodium hydrogen carbonate citric acid. When vinegar and baking soda are first mixed together hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. Web The bicarbonate reacts with hydrogen ions H from the citric acid to form carbon dioxide and water shown in Equation 1 of the Background section.
The daffodil plant grow best in soil having a pH range of 60 to65. Difference Between Sodium Carbonate And Sodium Bicarbonate. Web A conjugate acid within the BrønstedLowry acidbase theory is a chemical compound formed when an acid donates a proton H to a basein other words it is a base with a hydrogen ion added to it as in the reverse reaction it loses a hydrogen ion.
Hydrogen ion H known as a BrønstedLowry acid or forming a covalent bond with an electron pair known as a Lewis acid. Cefdinir for Oral Suspension USP after reconstitution contains 125 mg or 250 mg Cefdinir per 5 mL and the following inactive ingredients. Specifically this reaction involves acid-base chemistry since the baking sodaalso known as sodium bicarbonate NaHCO 3is a weak base and citric acid C 6 H 8 O 7 is a weak acid.
This can result in a metabolic alkalosis if the kidneys are unable to excrete the excess bicarbonate. The component ions in a salt compound can be either. Following is the comparison and difference.
SO2 Na2SO3 Na2S2O5. In flavoring drinks Carbonic acids d. Web C 14 H 13 N 5 O 5 S 2 H 2 O MW.
It is trisodium salt of citric acid and dissolves in water. Web The chemical structure of citric acid indicates that it is a triprotic acid and has three groups of carboxylic acid three ionizable atoms acidic hydrogen acid and three values of Ka pKa. Hydrochloric acids produces naturally in our stomach in the process of digestion in a certain amount if it exceeds then it causes acidity or ulcer.
Web Soda fizz comes from sodium bicarbonate and citric acid H 3C 6H 5O 7 reacting to make carbon dioxide sodium citrate Na 3C 6H 5O 7 and water. Web Ritalin may interact with blood thinners clonidine dobutamine epinephrine isoproterenol coldallergy medicine that contains phenylephrine a decongestant potassium citrate sodium acetate sodium bicarbonate citric acid and potassium citrate sodium citrate and citric acid medications to treat high or low blood pressure stimulant. The neutralization reaction with sodium hydroxide NaOH has a stoichiometry of 3 to 1 as shown by the balanced neutralization equation.
What Is The Reaction Between Baking Soda And Citric Acid Quora
Sodium Bicarbonate Karina Borunda
What Happened When Powdered Citric Acid And Powdered Sodium Bicarbonate Are Mixed Together Quora
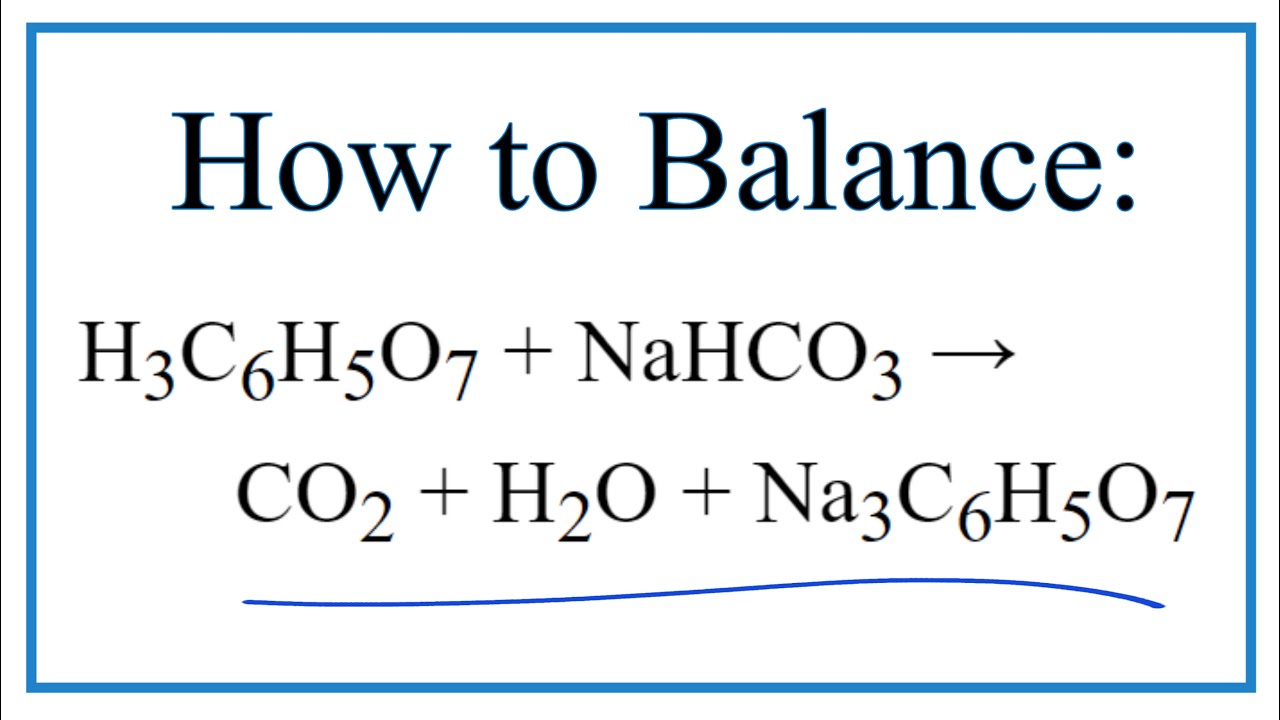
How To Balance H3c6h5o7 Nahco3 Co2 H2o Na3c6h5o7 Citric Acid Sodium Bicarbonate Youtube
0 Response to "Citric Acid and Sodium Bicarbonate Reaction Equation"
Post a Comment